
1-propanol is combusted to provide heat. the reaction and the enthalpy for the reaction are shown below. c3h7oh(l)+4.5o2(g)-> 3co2(g)+4h2o(l)deltah=-2,021kj below is a list of sentences that describe a chemical reaction. choose all of the sentences that apply to the above reaction. check all that apply. view available hint(s) check all that apply. the enthalpy for 2c3h7oh(l)+9o2(g)-> 6co2(g)+8h2o(l) is 4,042 kj this process is endothermic. this process is exothermic. the enthalpy for 2c3h7oh(l)+9o2(g)-> 6co2(g)+8h2o(l) is -4,042 kj the enthalpy for 2c3h7oh(l)+9o2(g)-> 6co2(g)+8h2o(l) is 2,021 kj this chemical reaction transfers heat from the surroundings to the system. the enthalpy for 2c3h7oh(g)+9o2(g)-> 6co2(g)+8h2o(l) is -2,021 kj this chemical reaction transfers heat from the system to the surroundings.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2
You know the right answer?
1-propanol is combusted to provide heat. the reaction and the enthalpy for the reaction are shown be...
Questions


Mathematics, 11.02.2021 14:00



English, 11.02.2021 14:00


Mathematics, 11.02.2021 14:00




Mathematics, 11.02.2021 14:00


Physics, 11.02.2021 14:00

Mathematics, 11.02.2021 14:00






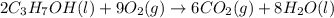 is 4,042 kJ , This process is exothermic and This chemical reaction transfers heat from the system to the surroundings.
is 4,042 kJ , This process is exothermic and This chemical reaction transfers heat from the system to the surroundings.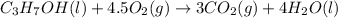
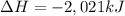
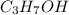 undergoes combustion to release 2,021kJ of heat.
undergoes combustion to release 2,021kJ of heat.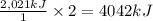 of heat.
of heat.

