
Chemistry, 26.06.2019 11:30 tiffanydowell13
Iron(iii) oxide is formed when iron combines with oxygen in the air. how many moles of fe2o3 are formed when 55.8 g or fe reacts completely with oxygen? 4fe(s)+3o 2(g) --> 2fe2o3(s) a. 0.25 mol b. 0. 50 mol c. o. 75 mol d. 1.00 mol

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 02:20
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 03:30
If you have 5.25 grams of methane (ch4), how many grams of co2 will you produce ?
Answers: 1

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2
You know the right answer?
Iron(iii) oxide is formed when iron combines with oxygen in the air. how many moles of fe2o3 are for...
Questions

Mathematics, 28.01.2020 05:31


History, 28.01.2020 05:31


History, 28.01.2020 05:31


English, 28.01.2020 05:31


Mathematics, 28.01.2020 05:31


Mathematics, 28.01.2020 05:31

Social Studies, 28.01.2020 05:31

Mathematics, 28.01.2020 05:31






Mathematics, 28.01.2020 05:31

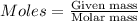
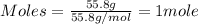
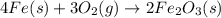
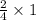 = 0.5 mole of Iron (III) oxide.
= 0.5 mole of Iron (III) oxide.

