
An ion of an isotope has a 2+ charge, an atomic mass of 56.9397 amu, 2 electrons at the n=4 energy level and 13 electrons at the n=3 energy level. determine a. atomic number b. mass number c. total number of electrons d. total number of s electrons e. total number of p electrons f. total number of d electrons

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2

Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2
You know the right answer?
An ion of an isotope has a 2+ charge, an atomic mass of 56.9397 amu, 2 electrons at the n=4 energy l...
Questions


Mathematics, 07.07.2019 20:00


Mathematics, 07.07.2019 20:00

Mathematics, 07.07.2019 20:00

Biology, 07.07.2019 20:00

World Languages, 07.07.2019 20:00

Mathematics, 07.07.2019 20:00



English, 07.07.2019 20:00



Mathematics, 07.07.2019 20:00




Biology, 07.07.2019 20:00

History, 07.07.2019 20:00

History, 07.07.2019 20:00

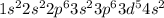
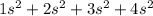 = 8
= 8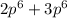 = 12
= 12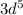 = 5
= 5

