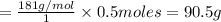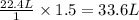Chemistry, 26.06.2019 14:30 BrodsterBj
You do an experiment in which you need 0.5 moles of tyrosine (c9h11no3). how many grams must you weigh out? 181g 272g 65g 90.5g what is the volume of 1.5 moles of gas stp? 20.0 l 9.02 l 33.6 l 22.4 l

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1


Chemistry, 22.06.2019 21:30
Athe top of a hill, an athlete on a skateboard has x joules of mechanical energy. how much mechanical energy will she have at the bottom of the hill? ignore the effects of friction.
Answers: 1

Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1
You know the right answer?
You do an experiment in which you need 0.5 moles of tyrosine (c9h11no3). how many grams must you wei...
Questions


History, 09.10.2019 01:40

Mathematics, 09.10.2019 01:40

Computers and Technology, 09.10.2019 01:40

Chemistry, 09.10.2019 01:40


Spanish, 09.10.2019 01:40

Mathematics, 09.10.2019 01:40

Physics, 09.10.2019 01:40

History, 09.10.2019 01:40

Chemistry, 09.10.2019 01:40



Mathematics, 09.10.2019 01:40




Biology, 09.10.2019 01:40

Biology, 09.10.2019 01:40

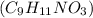 = 181 g/mol
= 181 g/mol