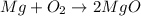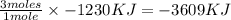Chemistry, 26.06.2019 15:00 trinity7265
Magnesium oxidizes via the reaction: 2 mg + o2 → 2 mgo the reaction has a △hrxn = -1203 kj. how much heat (in kj) is released when you completely react 3.000 moles of o2?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 11:00
Which are examples of how technology has advanced scientific understanding.1using hot water to sterilize medical equipment.2transplanting a human organ into another individual.3inserting genes from one sheep into another cell to make a cloneunderstanding the different structures that make up a cell.4examining microorganisms from the deepest parts of the ocean
Answers: 2

Chemistry, 22.06.2019 18:30
How many moles of lead are in 1.50 x 10^12 atoms of lead? could you explain the answer as well and not just give it to me i am refreshing for finals and i need to know how to do it
Answers: 3

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1
You know the right answer?
Magnesium oxidizes via the reaction: 2 mg + o2 → 2 mgo the reaction has a △hrxn = -1203 kj. how muc...
Questions

Mathematics, 13.01.2021 02:50

Computers and Technology, 13.01.2021 02:50


Arts, 13.01.2021 02:50

Mathematics, 13.01.2021 02:50

Arts, 13.01.2021 02:50


English, 13.01.2021 02:50






Mathematics, 13.01.2021 02:50


Mathematics, 13.01.2021 02:50

Mathematics, 13.01.2021 02:50

English, 13.01.2021 02:50


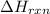 = -1230 KJ
= -1230 KJ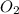 = 3 moles
= 3 moles