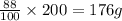1.you have a 2.0m nacl stock solution available. what is the volume you must dilute to make 500 ml of a 0.50m nacl solution? 2. how many grams of nano3 will precipitate if a saturated solution of nano3 in 200 g of water at 50°c is cooled to 20oc? assume the following solubility values for nano3: 114.0g/100g h2o at 50oc; 88.0g/100g h2o at 20oc 3. write equations to show how these substances ionize or dissociate in water. nh4cl cu(no3)2 hc2h3o2 hgcl2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 11:40
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3


Chemistry, 22.06.2019 23:30
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3
You know the right answer?
1.you have a 2.0m nacl stock solution available. what is the volume you must dilute to make 500 ml o...
Questions

Mathematics, 03.09.2020 20:01

History, 03.09.2020 20:01


Mathematics, 03.09.2020 20:01





Mathematics, 03.09.2020 20:01



Mathematics, 03.09.2020 20:01

English, 03.09.2020 20:01


Mathematics, 03.09.2020 20:01

Mathematics, 03.09.2020 20:01


Mathematics, 03.09.2020 20:01


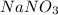 precipitate will be, 52 grams
precipitate will be, 52 grams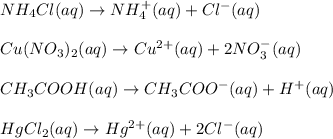
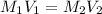
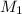 = concentration of NaCl stock solution = 2 M = 2 mole/L
= concentration of NaCl stock solution = 2 M = 2 mole/L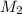 = concentration of NaCl solution = 0.50 M = 0.50 mole/L
= concentration of NaCl solution = 0.50 M = 0.50 mole/L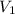 = volume of NaCl stock solution
= volume of NaCl stock solution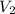 = volume of NaCl solution = 500 ml
= volume of NaCl solution = 500 ml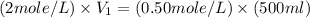
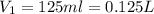 (1 L = 1000 ml)
(1 L = 1000 ml)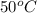 .
.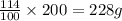
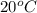 .
.