
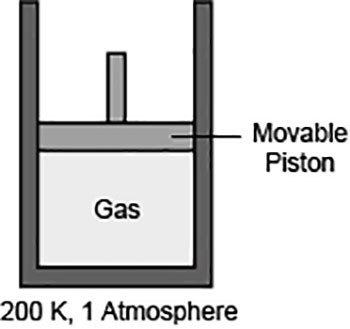
1. the pressure and temperature of a gas are held constant. which of the following is true for the volume of the gas? a.) it is inversely proportional to the number of moles of the gas. b.) it is directly proportional to the number of moles of the gas. c.) it is equal to the number of moles of the gas. d.) it is half of the number of moles of the gas.2. the diagram shows a gas in a container fitted with a movable piston. **picture shown** what will happen if the pressure of the system is raised to 1.5 atmospheres and the temperature remains same? a.) the piston will move upwards because the number of moles of the gas particles will increase. b.) the piston will move downwards because the gas particles will occupy less volume than before. c.) the piston will move upwards because the gas particles will hit the walls of the container with increased force. d.) the piston will move downwards because the gas particles will start moving randomly in all directions, pulling the piston down.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 07:20
Part b: study of equilibrium on solubility: mg(oh)2(s) ⇌ mg2+(aq) + 2 oh–(aq) cloudy clear (pink) 7. a. b. 8. a. b. 9. 10. 11. 12. when adding concentrated hydrochloric acid, how did the appearance of the equilibrium mixture change? the change in appearance indicated a shift in the point of equilibrium. in which direction did the equilibrium shift? (l) left (r) right explain your answer to question 7a. you should indicate which ion was added to or removed from the equilibrium mixture. when adding edta, how did the appearance of the equilibrium mixture change? the change in appearance indicated a shift in the point of equilibrium. in which direction did the equilibrium shift? (l) left (r) right explain your answer to question 8a. you should indicate which ion was added to or removed from the equilibrium mixture. upon heating in which direction is the equilibrium shifting? upon cooling in which direction is the equilibrium shifting? is the forward reaction a. endothermic explain your answers to questions 9, 10, and 11. (l) left (r) right (l) left (r) right b. exothermic
Answers: 1

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2
You know the right answer?
1. the pressure and temperature of a gas are held constant. which of the following is true for the v...
Questions




Mathematics, 28.01.2020 14:06

Chemistry, 28.01.2020 14:06

Chemistry, 28.01.2020 14:06

Social Studies, 28.01.2020 14:06

Mathematics, 28.01.2020 14:06

Spanish, 28.01.2020 14:06

Mathematics, 28.01.2020 14:06


History, 28.01.2020 14:06

English, 28.01.2020 14:06

Mathematics, 28.01.2020 14:06

Mathematics, 28.01.2020 14:06

History, 28.01.2020 14:06



Chemistry, 28.01.2020 14:06

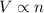 (At constant temperature and pressure)
(At constant temperature and pressure)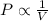 (At constant temperature and number of moles)
(At constant temperature and number of moles)

