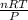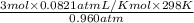1. what is the mass of 22.4 l of h2 at stp? a.) 1.01 grams b.) 2.02 grams c.) 11.2 grams d.) 22.4 grams 2. read the chemical equation. mg + hcl → mgcl2 + h2 how many liters of hydrogen gas is produced at 298 k and 0.960 atm if 3.00 moles of hydrochloric acid react with an excess of magnesium metal? a.) 38.2 liters b.) 42.6 liters c.) 66.7 liters d.) 76.4 liters

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1

Chemistry, 23.06.2019 03:30
The semi-conductors on the periodic table are classified as
Answers: 1
You know the right answer?
1. what is the mass of 22.4 l of h2 at stp? a.) 1.01 grams b.) 2.02 grams c.) 11.2 grams d.) 22.4 g...
Questions

World Languages, 25.10.2021 01:00

English, 25.10.2021 01:00



Biology, 25.10.2021 01:00




Advanced Placement (AP), 25.10.2021 01:00

History, 25.10.2021 01:00


History, 25.10.2021 01:00

Mathematics, 25.10.2021 01:00


Mathematics, 25.10.2021 01:00



Biology, 25.10.2021 01:00

Law, 25.10.2021 01:00

History, 25.10.2021 01:00

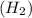 is 2.02 g/mol.
is 2.02 g/mol. 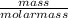
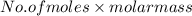
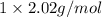
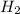 at STP is 2.02 grams.
at STP is 2.02 grams.