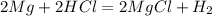Chemistry, 26.06.2019 17:00 catherinesquitieri
1. if you were to measure the mass of magnesium and hydrochloric acid before combining them in the test tube, how would that mass compare to the mass of reactants left in the test tube after the reaction? explain your answer and how it corresponds to the law of conservation of mass.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 22.06.2019 21:30
How can the periodic table be used to predict the behavior of elements?
Answers: 1

Chemistry, 23.06.2019 00:00
If many scientists conduct the same or similar experiments, and all obtain similar results, a can be written, which is a generally agreed-upon statement that explains and predicts how a natural phenomenon works.
Answers: 1

Chemistry, 23.06.2019 01:00
If a straight-chain hydrocarbon is a gas at room temperature, how many carbon atoms will it have? a. 6 carbon atoms b. 12 carbon atoms c. 24 carbon atoms d. 3 carbon atoms
Answers: 1
You know the right answer?
1. if you were to measure the mass of magnesium and hydrochloric acid before combining them in the t...
Questions


Mathematics, 31.03.2021 02:20

Physics, 31.03.2021 02:20


Mathematics, 31.03.2021 02:20




Computers and Technology, 31.03.2021 02:20





History, 31.03.2021 02:20




Mathematics, 31.03.2021 02:20


Arts, 31.03.2021 02:20