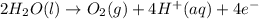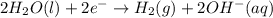Chemistry, 26.06.2019 19:30 michael2737
How does the electrolysis of water produce hydrogen gas? hydrogen cations give electrons to the anode through reduction reactions. hydrogen cations give electrons to the anode through oxidation reactions. electrons from the cathode combine with hydrogen cations through reduction reactions. electrons from the cathode combine with hydrogen cations through oxidation reactions.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 23:00
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1

Chemistry, 23.06.2019 05:00
1. true or false: minerals are inorganic. true false 2. inorganic means that something has never been found alive 3. halite is another name for and is a mineral with a cubic crystal pattern. table salt rock salt
Answers: 1

You know the right answer?
How does the electrolysis of water produce hydrogen gas? hydrogen cations give electrons to the ano...
Questions



Mathematics, 10.02.2021 20:40

History, 10.02.2021 20:40

Mathematics, 10.02.2021 20:40





Biology, 10.02.2021 20:40


Mathematics, 10.02.2021 20:40

Biology, 10.02.2021 20:40


History, 10.02.2021 20:40

Biology, 10.02.2021 20:40




Geography, 10.02.2021 20:40