Chemistry, 26.06.2019 20:30 irenecupcake4348
Which statement is correct about the reaction that occurs between calcium chloride and sodium carbonate? it is a single replacement reaction because two new compounds of calcium and sodium are formed. it is a double replacement reaction because calcium chloride and sodium carbonate exchange their non-metallic parts. it is a single replacement reaction because calcium replaces sodium within a compound as sodium is higher than calcium in the activity series. it is a double replacement reaction because the ions of calcium and sodium exchange places in an aqueous solution as calcium is higher than sodium in the activity series.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:30
As a part of an experiment a student burns propane to produce carbon dioxide and water she remembers that she must follow the law conservation of matter when writing a balanced chemical equation which of these equation adheres to the law of conservation of matter
Answers: 1

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 20:30
Which of the following is not true about the atomic model of substances?
Answers: 1
You know the right answer?
Which statement is correct about the reaction that occurs between calcium chloride and sodium carbon...
Questions

Social Studies, 18.09.2021 03:20

Computers and Technology, 18.09.2021 03:20






Mathematics, 18.09.2021 03:20

Biology, 18.09.2021 03:20

English, 18.09.2021 03:20




Engineering, 18.09.2021 03:20




History, 18.09.2021 03:30


Chemistry, 18.09.2021 03:30

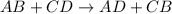
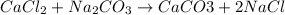
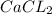 are reactants
are reactants 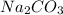 and form products by exchanging their nonmetallic parts. The products obtained in the reaction are
and form products by exchanging their nonmetallic parts. The products obtained in the reaction are 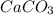 and
and