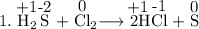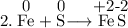Chemistry, 26.06.2019 20:30 wlackey2020
Read the following chemical equations. reaction 1: h2s + cl2 → 2hcl + s reaction 2: fe + s → fes which of the following statements is true for both the chemical equations? a. chlorine is reduced in reaction 1 and iron is reduced in reaction 2. b. chlorine is oxidized in reaction 1 and iron is oxidized in reaction 2. c. chlorine is oxidized in reaction 1 and iron is reduced in reaction 2. d. chlorine is reduced in reaction 1 and iron is oxidized in reaction 2.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Embryos of different species look very similar, which shows that the organisms share a ancestor.
Answers: 1

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1
You know the right answer?
Read the following chemical equations. reaction 1: h2s + cl2 → 2hcl + s reaction 2: fe + s → fes...
Questions

Social Studies, 18.10.2020 09:01

Mathematics, 18.10.2020 09:01

English, 18.10.2020 09:01

Health, 18.10.2020 09:01

History, 18.10.2020 09:01

Computers and Technology, 18.10.2020 09:01



Mathematics, 18.10.2020 09:01

Mathematics, 18.10.2020 09:01

History, 18.10.2020 09:01

Mathematics, 18.10.2020 09:01

Mathematics, 18.10.2020 09:01



Mathematics, 18.10.2020 09:01

English, 18.10.2020 09:01

Mathematics, 18.10.2020 09:01

History, 18.10.2020 09:01

German, 18.10.2020 09:01