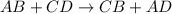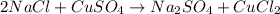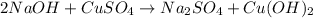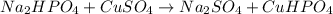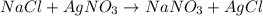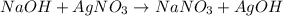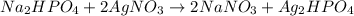Chemistry, 26.06.2019 22:30 greenbyron88
Me for part 3: double-displacement reactions: for each of the double-displacement reactions, describe what happened in each well, including the colors of any products that formed. if a chemical reaction occurred, write a balanced equation for it. then using the a, b symbols, write a general equation for a double-displacement reaction. here are the chemical formulas of the reactants for each reaction: • sodium chloride – nacl copper sulfate – cuso4 • sodium hydroxide – naoh copper sulfate – cuso4 • sodium phosphate – na2hpo4 copper sulfate – cuso4 • sodium chloride – nacl silver nitrate – agno3 • sodium hydroxide – naoh silver nitrate – agno3 • sodium phosphate – na2hpo4 silver nitrate – agno3

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

Chemistry, 23.06.2019 11:00
Suppose you increase your walking speed from 7 m/s to 15 m/s in a period of 1 s. what is your acceleration?
Answers: 1

Chemistry, 23.06.2019 11:30
If this sedimentary rock layer is truly the oldest one of marine origin, what do you think that tells usabout the formation of earth's oceans?
Answers: 2

Chemistry, 23.06.2019 16:00
Which part of the mantle is similar to the crust ? (science)
Answers: 3
You know the right answer?
Me for part 3: double-displacement reactions: for each of the double-displacement reactions, descr...
Questions

Biology, 18.07.2019 22:30

Biology, 18.07.2019 22:30


History, 18.07.2019 22:30

Chemistry, 18.07.2019 22:30


History, 18.07.2019 22:30



History, 18.07.2019 22:30

History, 18.07.2019 22:30

Biology, 18.07.2019 22:30

Mathematics, 18.07.2019 22:30


Social Studies, 18.07.2019 22:30

History, 18.07.2019 22:30


History, 18.07.2019 22:30