
What is the molarity of solution obtained when 5.71 g of sodium carbonate-10-water is dissolved in water and made up to 250.0 cm^3 solution? (relative atomic masses: h = 1.0, c = 12.0, o = 16.0, na = 23.0) a. 0.08 mol dm^-3 b. 0.11 mol dm^-3 c. 0.16 mol dm^-3 d. 0.22 mol dm^-3

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 22.06.2019 22:30
What is the work done by the electric force to move a 1 c charge from a to b?
Answers: 2

Chemistry, 23.06.2019 00:30
Balance the following reaction. as2s3 + 9o2 → 2as2o3 + so2
Answers: 2
You know the right answer?
What is the molarity of solution obtained when 5.71 g of sodium carbonate-10-water is dissolved in w...
Questions

Social Studies, 14.04.2020 21:26










History, 14.04.2020 21:26


Chemistry, 14.04.2020 21:26

Mathematics, 14.04.2020 21:26






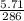 = 0.0199 moles of Na₂CO₃.10 H₂O. So, 0.0199 moles of Na₂CO₃.10 H₂O present in 250 cm³ volume of solution.
= 0.0199 moles of Na₂CO₃.10 H₂O. So, 0.0199 moles of Na₂CO₃.10 H₂O present in 250 cm³ volume of solution.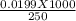 = 0.0796 moles. So, the molarity of the solution is 0.0796 mol/dm³ ≅ 0.08 mol/dm³
= 0.0796 moles. So, the molarity of the solution is 0.0796 mol/dm³ ≅ 0.08 mol/dm³

