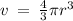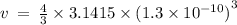Chemistry, 27.06.2019 02:00 msbanks317
Given the atomic radius of xenon, 1.3 ? , and knowing that a sphere has a volume of 4? r3/3, calculate the fraction of space that xe atoms occupy in a sample of xenon at stp.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2

Chemistry, 22.06.2019 23:00
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2
You know the right answer?
Given the atomic radius of xenon, 1.3 ? , and knowing that a sphere has a volume of 4? r3/3, calcula...
Questions





Mathematics, 25.08.2019 04:10



Computers and Technology, 25.08.2019 04:10



Physics, 25.08.2019 04:10


English, 25.08.2019 04:10




Biology, 25.08.2019 04:10


History, 25.08.2019 04:10

English, 25.08.2019 04:10