
Gerald's science teacher mixed room temperature samples of hydrochloric acid and sodium hydroxide in a large beaker. the solution still looked clear like water, but when the students carefully touched the beaker one at a time, it felt warm to the touch. why did the beaker most likely feel warm? a. the two liquids were not soluble in water. b. the release of a gas heated the solution. c. the energy of mixing warmed the solution. d. a chemical reaction was producing a new substance.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1

Chemistry, 22.06.2019 03:30
Each pair of clay balls represents to planetesimals if each plane test molluscum pound of the same material and is separated by the same distance which pair experiences the greatest gravitational attraction
Answers: 2

Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1
You know the right answer?
Gerald's science teacher mixed room temperature samples of hydrochloric acid and sodium hydroxide in...
Questions






Mathematics, 24.07.2020 09:01





Mathematics, 24.07.2020 09:01





Physics, 24.07.2020 09:01

Mathematics, 24.07.2020 09:01



Mathematics, 24.07.2020 09:01


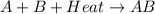
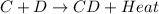 is an exothermic reaction.
is an exothermic reaction.

