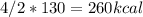Chemistry, 27.06.2019 15:30 hahalol123goaway
Calculate the enthalpy change when 4.00 mol cl2o7 is produced according to the following balanced equation: 2cl2(g) + 7o2(g) + 130kcal -> 2cl2o7(g) a. 1040 kcal b. -260 kcal c. 260 kcal d. -1040 kcal ** if you could explain it as well, that would be much appreciated if not, thats okay too its multiple choice

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3

Chemistry, 22.06.2019 23:00
What prefix multiplier is appropriate for reporting a measurement of 5.57 ×10−5 m?
Answers: 1
You know the right answer?
Calculate the enthalpy change when 4.00 mol cl2o7 is produced according to the following balanced eq...
Questions


English, 02.09.2019 07:30


Advanced Placement (AP), 02.09.2019 07:30




Mathematics, 02.09.2019 07:30

History, 02.09.2019 07:30


Biology, 02.09.2019 07:30

History, 02.09.2019 07:30

History, 02.09.2019 07:30


History, 02.09.2019 07:30

Physics, 02.09.2019 07:30




Chemistry, 02.09.2019 07:30

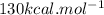
 is mentioned because it is for per mole of reaction. So for 4. moles of the product
is mentioned because it is for per mole of reaction. So for 4. moles of the product  we need 4/2 moles of reaction to be used to calculate associated enthalphy change for the reaction.
we need 4/2 moles of reaction to be used to calculate associated enthalphy change for the reaction.