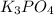Chemistry, 31.01.2020 17:47 genyjoannerubiera
Sodium metal reacts with water to produce hydrogen gas.
what best describes this reaction?
a•a single replacement reaction takes place because sodium is less reactive than hydroxide ions.
b•a double replacement reaction takes place because sodium is less reactive than hydroxide ions.
c•a double replacement reaction takes place because sodium is more reactive than hydrogen.
d•a single replacement reaction takes place because sodium is more reactive than hydrogen.
question 2(multiple choice worth 4 points)
(04.03 mc)
the table shows the nature of reactants and products formed in a certain type of chemical reaction.
nature of reactants and products
reactants products
metal + ionic compound metal + ionic compound
which of the following is true about the type of chemical reaction?
a•it is a single replacement reaction, and the anions in the two ionic compounds are different.
b•it is a single replacement reaction, and the cations in the two ionic compounds are different.
c•it is a double replacement reaction, and the anions in the two ionic compounds are different.
d•it is a double replacement reaction, and the cations in the two ionic compounds are different.
question 3(multiple choice worth 4 points)
(04.03 lc)
which of the following is a single replacement reaction?
a• ba(oh)2 + h2so4 → baso4 + 2h2o
b• 2mg + o2 → 2mgo
c• h2o+ co2 → h2co3
d• zn + h2so4 → znso4 + h2
question 4 (true/false worth 2 points)
(04.03 lc)
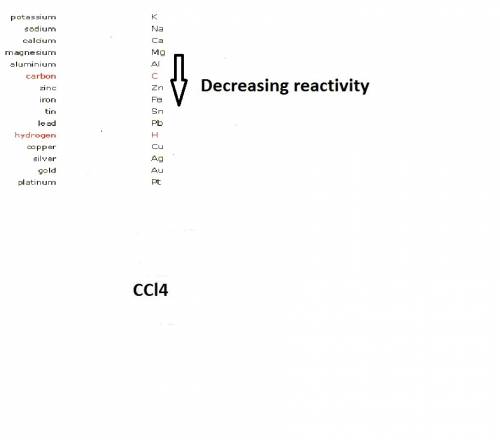
when cacl2 and zn react together, zinc (zn) can replace chlorine (cl) in the compound because zinc is higher on the periodic table.
true
false
question 5 (true/false worth 2 points)
(04.03 lc)
a double replacement reaction is a reaction in which one element replaces a similar element within a compound.
true
false
question 6(multiple choice worth 4 points)
(04.03 mc)
which of the following equations has the correct products and is balanced correctly for a reaction between na3po4 and koh?
a• na3po4 + 3koh → 3naoh + k3po4, because k retains the same charge throughout the reaction
b• na3po4 + koh → na3oh + kpo4, because k increases in charge from 1+ to 3+ when it is replaced
c• na3po4 + koh → 3naoh + k3po4, because k retains the same charge throughout the reaction
d• na3po4 + koh → na3oh + k3po4, because k increases in charge from 1+ to 3+ when it is replaced

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:10
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 23.06.2019 02:20
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2
You know the right answer?
Sodium metal reacts with water to produce hydrogen gas.
what best describes this reactio...
what best describes this reactio...
Questions

Mathematics, 24.11.2020 04:40

Health, 24.11.2020 04:40


Mathematics, 24.11.2020 04:40


Mathematics, 24.11.2020 04:40

Mathematics, 24.11.2020 04:40

Social Studies, 24.11.2020 04:40


Health, 24.11.2020 04:40


History, 24.11.2020 04:40

History, 24.11.2020 04:40





Mathematics, 24.11.2020 04:40


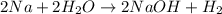 Sodium metal reacts with water to givesodium hydroxide and hydrogen gas. A single replacement reaction takes place because sodium is more reactive than hydrogen.
Sodium metal reacts with water to givesodium hydroxide and hydrogen gas. A single replacement reaction takes place because sodium is more reactive than hydrogen. 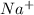 and
and 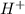 get reduced to give
get reduced to give 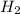 .
.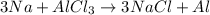 where Sodium is a metal and
where Sodium is a metal and 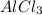 is an ionic compound. Na being more reactive than Al, displaces it from its salt solution.
is an ionic compound. Na being more reactive than Al, displaces it from its salt solution.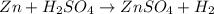
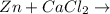 no reaction
no reaction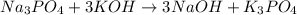 , because K retains the same charge throughout the reaction
, because K retains the same charge throughout the reaction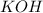 as well as
as well as