
Chemistry, 27.06.2019 19:30 ccarwile01
Determine the rate law, including the values of the orders and rate law constant, for the following reaction using the experimental data provided. (4 points) a + b yieldsproducts trial [a] [b] rate 1 0.10 m 0.20 m 1.2 × 10-2 m/min 2 0.10 m 0.40 m 4.8 × 10-2 m/min 3 0.20 m 0.40 m 9.6 × 10-2 m/min

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1


Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3

Chemistry, 23.06.2019 01:00
Which is true concerning the products and reactants of photosynthesis and cellular respiration? a. the products of photosynthesis are sugars and the reactants of cellular respiration are starches. b. the products of photosynthesis are reactants in cellular respiration. c. oxygen is needed for photosynthesis and is given off in cellular respiration.
Answers: 2
You know the right answer?
Determine the rate law, including the values of the orders and rate law constant, for the following...
Questions

Advanced Placement (AP), 18.09.2019 15:50


Biology, 18.09.2019 15:50



Mathematics, 18.09.2019 15:50

History, 18.09.2019 15:50








Social Studies, 18.09.2019 15:50

History, 18.09.2019 15:50


History, 18.09.2019 15:50

Mathematics, 18.09.2019 15:50

![k[A]^1[B]^2](/tpl/images/0024/4376/f6c70.png) , order with respect to A is 1, order with respect to B is 2 and total order is 3. Rate law constant is
, order with respect to A is 1, order with respect to B is 2 and total order is 3. Rate law constant is 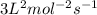
![Rate=k[A]^x[B]^y](/tpl/images/0024/4376/ddde1.png)
![1.2\times 10^{-2}=k[0.10]^x[0.20]^y](/tpl/images/0024/4376/c938a.png) (1)
(1)![4.8\times 10^{-2}=k[0.10]^x[0.40]^y](/tpl/images/0024/4376/a7ba9.png) (2)
(2)![\frac{4.8\times 10^{-2}}{1.2\times 10^{-2}}=\frac{k[0.10]^x[0.40]^y}{k[0.10]^x[0.20]^y}](/tpl/images/0024/4376/b4c1f.png)
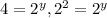 therefore y=2.
therefore y=2.![9.6\times 10^{-2}=k[0.20]^x[0.40]^y](/tpl/images/0024/4376/18d47.png) (4)
(4)![\frac{9.6\times 10^{-2}}{4.8\times 10^{-2}}=\frac{k[0.20]^x[0.40]^y}{k[0.10]^x[0.40]^y}](/tpl/images/0024/4376/61313.png)
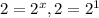 , x=1
, x=1![Rate=k[A]^1[B]^2](/tpl/images/0024/4376/ca297.png)
![1.2\times 10^{-2}=k[0.10]^1[0.20]^2](/tpl/images/0024/4376/f6a0d.png)
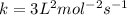 .
.

