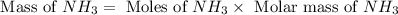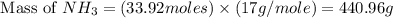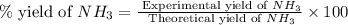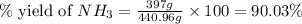Chemistry, 27.06.2019 20:30 elijahlylejamez45
25 i got the #1, just not #2 and #3. an industrial chemical company has opened a new plant that will produce ammonia (nh3). hydrogen and nitrogen gases are reacted to produce the ammonia. for the first batch of ammonia production, 475 g of nitrogen is reacted with excess hydrogen, and 397 g of ammonia are produced. • write the balanced equation for the formation of ammonia from hydrogen and nitrogen.  2nh3 • calculate the theoretical yield of ammonia. work must be shown to earn credit. • calculate the percent yield for the ammonia production. work must be shown to earn credit.
2nh3 • calculate the theoretical yield of ammonia. work must be shown to earn credit. • calculate the percent yield for the ammonia production. work must be shown to earn credit.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

You know the right answer?
25 i got the #1, just not #2 and #3. an industrial chemical company has opened a new plant that wil...
Questions

Mathematics, 11.09.2019 00:30

History, 11.09.2019 00:30




Mathematics, 11.09.2019 00:30

Mathematics, 11.09.2019 00:30









History, 11.09.2019 00:30

SAT, 11.09.2019 00:30

English, 11.09.2019 00:30


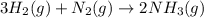
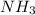 gas = 440.96 g
gas = 440.96 g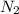 = 475 g
= 475 g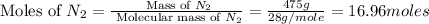
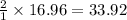 moles of
moles of