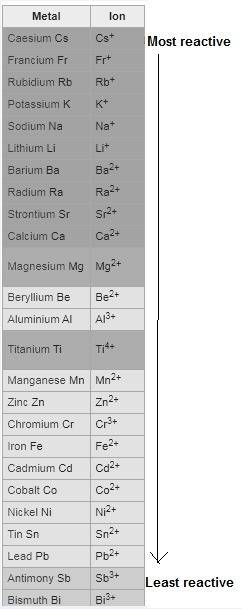
Question 1 (true/false worth 2 points) (04.03 lc) when pb and alcl3 react together, lead (pb) can replace aluminum (al) in the compound because lead is lower on the activity series. true false question 2(multiple choice worth 4 points) (04.03 mc) which of the following equations has the correct products and is balanced correctly for a reaction between na3po4 and koh? na3po4 + 3koh → 3naoh + k3po4, because k retains the same charge throughout the reaction na3po4 + koh → na3oh + kpo4, because k increases in charge from 1+ to 3+ when it is replaced na3po4 + koh → 3naoh + k3po4, because k retains the same charge throughout the reaction na3po4 + koh → na3oh + k3po4, because k increases in charge from 1+ to 3+ when it is replaced question 3(multiple choice worth 4 points) (04.03 lc) which of the following is a single replacement reaction? ba(oh)2 + h2so4 → baso4 + 2h2o 2mg + o2 → 2mgo h2o+ co2 → h2co3 zn + h2so4 → znso4 + h2 question 4(multiple choice worth 4 points) (04.03 mc) sodium metal reacts with water to produce hydrogen gas. what best describes this reaction? a single replacement reaction takes place because sodium is less reactive than hydroxide ions. a double replacement reaction takes place because sodium is less reactive than hydroxide ions. a double replacement reaction takes place because sodium is more reactive than hydrogen. a single replacement reaction takes place because sodium is more reactive than hydrogen. question 5 (true/false worth 2 points) (04.03 lc) a single replacement reaction is a reaction in which one element replaces a similar element within a compound. true false question 6(multiple choice worth 4 points) (04.03 mc) the table shows the nature of reactants and products formed in a certain type of chemical reaction. nature of reactants and products reactants products metal + ionic compound metal + ionic compound which of the following is true about the type of chemical reaction? it is a single replacement reaction, and the anions in the two ionic compounds are different. it is a single replacement reaction, and the cations in the two ionic compounds are different. it is a double replacement reaction, and the anions in the two ionic compounds are different. it is a double replacement reaction, and the cations in the two ionic compounds are different.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Order the following from smallest to largest atom, electron, quark, proton, neutron, molecule, nucleus
Answers: 1

Chemistry, 21.06.2019 20:30
What problem would a person have if the nucleic acid in one of his or her cells were damaged?
Answers: 2

Chemistry, 21.06.2019 21:00
Which of the following compounds does not contain molecules? question 2 options: co2 h2 nacl h2o
Answers: 1

Chemistry, 21.06.2019 23:00
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3
You know the right answer?
Question 1 (true/false worth 2 points) (04.03 lc) when pb and alcl3 react together, lead (pb) can re...
Questions

Mathematics, 10.06.2020 22:57


Chemistry, 10.06.2020 22:57



Mathematics, 10.06.2020 22:57









Mathematics, 10.06.2020 22:57

Mathematics, 10.06.2020 22:57

Mathematics, 10.06.2020 22:57

Mathematics, 10.06.2020 22:57


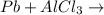 no reaction
no reaction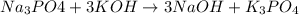 , because K retains the same charge throughout the reaction.
, because K retains the same charge throughout the reaction. 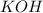 as well as
as well as 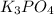
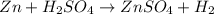
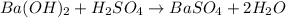 : is a double displacement reaction in which ion exchange takes place.
: is a double displacement reaction in which ion exchange takes place. 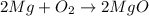 : It is a synthesis reaction as two reactants combine to give a single product.
: It is a synthesis reaction as two reactants combine to give a single product.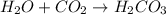 : It is a synthesis reaction as two reactants combine to give a single product.
: It is a synthesis reaction as two reactants combine to give a single product.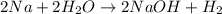 Sodium metal reacts with water to produce hydrogen gas: A single replacement reaction takes place because sodium is more reactive than hydrogen.
Sodium metal reacts with water to produce hydrogen gas: A single replacement reaction takes place because sodium is more reactive than hydrogen. 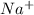 in NaOH and
in NaOH and 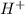 get reduced to give
get reduced to give 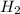
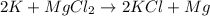 where K is a metal and
where K is a metal and 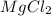 is an ionic compound. K being more reactive than Mg, displaces it from its salt solution.
is an ionic compound. K being more reactive than Mg, displaces it from its salt solution.