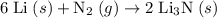Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2
You know the right answer?
Lithium and nitrogen react in a combination reaction to produce lithium nitride: 6li (s) + n2 (g) →...
Questions

Mathematics, 05.03.2021 15:20

Computers and Technology, 05.03.2021 15:20

Mathematics, 05.03.2021 15:20



Mathematics, 05.03.2021 15:20

Mathematics, 05.03.2021 15:20

Geography, 05.03.2021 15:20

Mathematics, 05.03.2021 15:20


English, 05.03.2021 15:20

English, 05.03.2021 15:20

Mathematics, 05.03.2021 15:20

Mathematics, 05.03.2021 15:20


English, 05.03.2021 15:20


Mathematics, 05.03.2021 15:20

Mathematics, 05.03.2021 15:20

Biology, 05.03.2021 15:20