
Chemistry, 28.06.2019 02:30 Austin4094
Potassium hydroxide (koh) reacts with sulfuric acid (h2so4) to produce potassium sulfate (k2so4) and water. what best describes this reaction? a single replacement reaction occurs because potassium ions bond with sulfate ions to form a salt. a double replacement reaction occurs because potassium ions bond with sulfate ions to form a salt. a single replacement reaction occurs because potassium is more reactive than hydrogen. a double replacement reaction occurs because potassium is less reactive than hydrogen.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3
You know the right answer?
Potassium hydroxide (koh) reacts with sulfuric acid (h2so4) to produce potassium sulfate (k2so4) and...
Questions

Mathematics, 10.03.2021 01:30

History, 10.03.2021 01:30

Mathematics, 10.03.2021 01:30

History, 10.03.2021 01:30



History, 10.03.2021 01:30



Mathematics, 10.03.2021 01:30



Mathematics, 10.03.2021 01:30

Mathematics, 10.03.2021 01:30


Mathematics, 10.03.2021 01:30


Mathematics, 10.03.2021 01:30

Social Studies, 10.03.2021 01:30

Mathematics, 10.03.2021 01:30


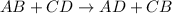
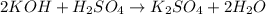
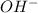 ion of KOH combines with
ion of KOH combines with 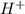 ion of sulfuric acid to give water.
ion of sulfuric acid to give water.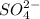 ) of sulfuric acid gets combine with two potassium ion (
) of sulfuric acid gets combine with two potassium ion (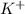 )to give salt of potassium sulfate,
)to give salt of potassium sulfate, 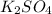 .
.

