
Chemistry, 28.06.2019 02:30 jobucks9976
Fe3+(aq) (yellow) + scn-(aq) (colorless) fescn2+(aq) (blood-red) chloride ions are colorless. potassium ions are also colorless. the above equilibrium can be created by mixing an iron (iii) chloride solution with a potassium thiocyanate solution. based on this information and the colors in the equilibrium above answer the first two questions. 1. what color would an fecl3 solution be? 2. what color would a kscn solution be? 3. what color do you get when you mix fecl3 and kscn as shown in the video for the control test tube?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Esign techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 3

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 12:00
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2

You know the right answer?
Fe3+(aq) (yellow) + scn-(aq) (colorless) fescn2+(aq) (blood-red) chloride ions are colorless. potass...
Questions

History, 05.11.2019 13:31



Mathematics, 05.11.2019 13:31







Social Studies, 05.11.2019 13:31


Mathematics, 05.11.2019 13:31

Biology, 05.11.2019 13:31


Biology, 05.11.2019 13:31



Chemistry, 05.11.2019 13:31

Physics, 05.11.2019 13:31

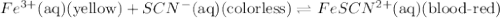
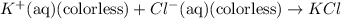
![FeCl_3+K(SCN)\rightleftharpoons KCl+[Fe(SCN)]Cl_2(blood-red)](/tpl/images/0025/5430/e3b23.png)
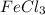 we will get blood red color solution of
we will get blood red color solution of ![[FeSCN]Cl_2](/tpl/images/0025/5430/ecffe.png) .
.

