
Chemistry, 28.06.2019 09:00 Zachary4759
The enthalpy change for converting 1.00 mol of ice at -25.0 ∘c to water at 90.0∘c is kj. the specific heats of ice, water, and steam are 2.09 j/g−k, 4.18 j/g−k, and 1.84 j/g−k, respectively. for h2o, δ hfus = 6.01kj/mol, and δhvap = 40.67 kj/mol.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
Adepositional also feature that forms where a stream enters a lake or an ocean is a
Answers: 2

Chemistry, 23.06.2019 03:00
What do electromagnetic waves carry? how are they produced through which media can they move? where do they transfer energy? what do they not transfer? what do mechanical waves carry? how are they produced? through which media can they move? where do they transfer energy? what do they not transfer?
Answers: 1

Chemistry, 23.06.2019 05:00
Asolution is made by dissolving 2.3 moles of sodium chloride (nacl) in 0.155 kilograms of water. if the molal boiling point constant for water (kb) is 0.51 °c/m, what would be the boiling point of this solution? show all the steps taken to solve this problem.
Answers: 1

Chemistry, 23.06.2019 10:00
Two moles of potassium chloride and three moles of oxygen are produced from the decomposition of two moles of potassium chlorate, kcos3(s). write the balanced equation. how many moles of oxygen are produced from 12 moles of potassium chlorate
Answers: 1
You know the right answer?
The enthalpy change for converting 1.00 mol of ice at -25.0 ∘c to water at 90.0∘c is kj. the specif...
Questions


Mathematics, 27.02.2021 05:50

Arts, 27.02.2021 05:50







Mathematics, 27.02.2021 05:50

Mathematics, 27.02.2021 05:50

Mathematics, 27.02.2021 05:50


Mathematics, 27.02.2021 05:50


Mathematics, 27.02.2021 05:50

Mathematics, 27.02.2021 05:50


Mathematics, 27.02.2021 05:50

English, 27.02.2021 05:50

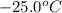 to water at
to water at 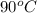 is, 7.712 KJ
is, 7.712 KJ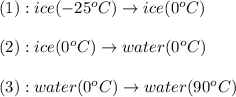
![\Delta H=[m\times c_{ice}\times (T_2-T_1)]+\Delta H_{fusion}+[m\times c_{water}\times (T_3-T_2)]](/tpl/images/0026/6160/eeaad.png)
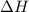 = enthalpy change
= enthalpy change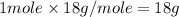
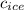 = specific heat of ice = 2.09 J/gk
= specific heat of ice = 2.09 J/gk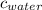 = specific heat of water = 4.18 J/gk
= specific heat of water = 4.18 J/gk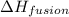 = enthalpy change for fusion = 6.01 KJ/mole = 0.00601 J/mole
= enthalpy change for fusion = 6.01 KJ/mole = 0.00601 J/mole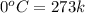
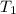 = initial temperature of ice =
= initial temperature of ice = 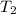 = final temperature of ice =
= final temperature of ice = 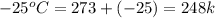
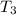 = initial temperature of water =
= initial temperature of water = 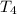 = final temperature of water =
= final temperature of water = 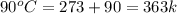
![\Delta H=[18g\times 2.09J/gK\times (273-248)k]+0.00601J+[18g\times 4.18J/gK\times (363-273)k]](/tpl/images/0026/6160/21744.png)
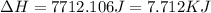 (1 KJ = 1000 J)
(1 KJ = 1000 J)

