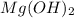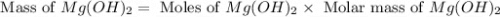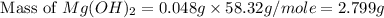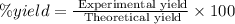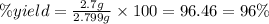Select the correct answer. excess sodium hydroxide is added to a solution containing 4.6 grams of magnesium chloride. a reaction takes place according to this equation: 2naoh(aq) + mgcl2(aq) → 2nacl(aq) + mg(oh)2(s). the magnesium hydroxide produced by the reaction was collected and weighed. if the mass of the magnesium hydroxide was 2.7 grams, what was the percent yield? use the periodic table. a. 48% b. 59% c. 61% d. 96%

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 00:30
There are approximately 15 milliliters (ml) in 1 tablespoon (tbsp). what expression can be used to find the approximate number of milliliters in 3 tbsp?
Answers: 1

Chemistry, 23.06.2019 03:30
Which of the following describes the entropy change as a solution is made from a liquid and solid
Answers: 1

Chemistry, 23.06.2019 11:20
The chemical composition of soil varies with depth. an article in communications in soil science and plant analysis describes chemical analyses of soil taken from a farm in western australia. fifty specimens were each taken at depths 50 and 250 cm. at a depth of 50 cm, the average no3 concentration (in mg/l) was 88.5 with a standard deviation of 49.4. at a depth of 250 cm, the average concentration was 110.6 with a standard deviation of 51.5. find a 95% confidence interval for the difference in no3 concentrations at the two depths.
Answers: 1

Chemistry, 23.06.2019 13:00
Write the balanced chemical reaction for the formation of fe2(so4)3 from fe2o3 and so3 and determine how many moles of fe2(so4)3 are formed when 12.7 mol of so3 are reacted.
Answers: 1
You know the right answer?
Select the correct answer. excess sodium hydroxide is added to a solution containing 4.6 grams of ma...
Questions



Mathematics, 06.03.2020 22:36








Biology, 06.03.2020 22:36





Mathematics, 06.03.2020 22:37

History, 06.03.2020 22:37


Geography, 06.03.2020 22:37

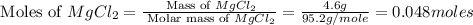
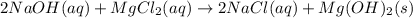
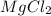 react to give 1 mole of
react to give 1 mole of