Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:20
Aluminum reacts with chlorine gas to form aluminum chloride via the following reaction: 2al(s)+3cl2(g)→2alcl3(s) what is the maximum mass of aluminum chloride that can be formed when reacting 32.0 g of aluminum with 37.0 g of chlorine? express your answer to three significant figures and include the appropriate units.
Answers: 2

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1
You know the right answer?
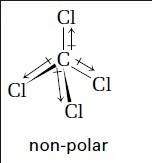
Analyze the bonds of ccl4. what is the shape of the ccl4 molecule? is it symmetrical? does this me...
Questions


Advanced Placement (AP), 23.04.2021 01:50

Mathematics, 23.04.2021 01:50







Mathematics, 23.04.2021 01:50


French, 23.04.2021 01:50


History, 23.04.2021 01:50

Mathematics, 23.04.2021 01:50