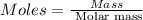Chemistry, 28.06.2019 17:30 kprincess16r
Ascorbic acid, or vitamin c (c6h8o6, molar mass = 176 g/mol), is a naturally occurring organic compound with antioxidant properties. a healthy adult’s daily requirement of vitamin c is 70-90 mg. a sweet lime contains 2.88×10−4 mol of ascorbic acid. to determine whether the ascorbic acid in a sweet lime meets the daily requirement, calculate the mass of ascorbic acid in 2.88×10−4 mol of ascorbic acid.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1
You know the right answer?
Ascorbic acid, or vitamin c (c6h8o6, molar mass = 176 g/mol), is a naturally occurring organic compo...
Questions

Mathematics, 22.09.2019 12:30




Social Studies, 22.09.2019 12:30

Health, 22.09.2019 12:30

History, 22.09.2019 12:30


Biology, 22.09.2019 12:30

History, 22.09.2019 12:30


English, 22.09.2019 12:30

Mathematics, 22.09.2019 12:30




Biology, 22.09.2019 12:30

Mathematics, 22.09.2019 12:30

English, 22.09.2019 12:30