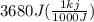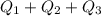Chemistry, 28.06.2019 18:00 nuconteaza119
Ethanol (c2h5oh) melts at -114â°c. the enthalpy of fusion is 5.02 kj/mol. the specific heats of solid and liquid ethanol are 0.97 j/gk and 2.3 j/gk, respectively. how much heat (kj) is needed to convert 25.0 g of solid ethanol at -135â°c to liquid ethanol at -50â°c?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:10
How is 0.00235 expressed in proper scientific notation? a. 2.35 × 10-3 b. 0.235 × 10-2 c. 2.35 d. 2.35 × 103
Answers: 1


Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3
You know the right answer?
Ethanol (c2h5oh) melts at -114â°c. the enthalpy of fusion is 5.02 kj/mol. the specific heats of soli...
Questions

History, 10.07.2019 00:30

Arts, 10.07.2019 00:30

Mathematics, 10.07.2019 00:30


Advanced Placement (AP), 10.07.2019 00:30





Mathematics, 10.07.2019 00:30

English, 10.07.2019 00:30

History, 10.07.2019 00:30

Mathematics, 10.07.2019 00:30

Mathematics, 10.07.2019 00:30

Physics, 10.07.2019 00:30

Mathematics, 10.07.2019 00:30

Mathematics, 10.07.2019 00:30


Chemistry, 10.07.2019 00:30

Computers and Technology, 10.07.2019 00:30

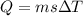
 is the change in temperature.
is the change in temperature.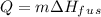
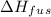 is the enthalpy of fusion.
is the enthalpy of fusion.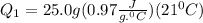
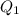 = 509.25 J
= 509.25 J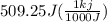
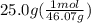
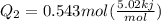
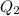 = 2.72 kj
= 2.72 kj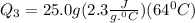
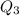 = 3680 J
= 3680 J