
In this reaction, what roll does the lead (ii) nitrate play when 50.0 ml of 0.100m iron (iii) chloride are mixed with 50.0 ml of 0.100m lead (ii) nitrate? a) lead (ii) nitrate increases the amount of precipitate. b) the reactant lead (ii) nitrate decreases product yield. c) lead (ii) nitrate is the excess reactant in the reaction. d) the lead (ii) nitrate is the reaction's limiting reactant.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1


Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3
You know the right answer?
In this reaction, what roll does the lead (ii) nitrate play when 50.0 ml of 0.100m iron (iii) chlori...
Questions


English, 29.05.2021 06:50

Mathematics, 29.05.2021 06:50


History, 29.05.2021 06:50



Mathematics, 29.05.2021 06:50


Mathematics, 29.05.2021 06:50


Mathematics, 29.05.2021 06:50

Law, 29.05.2021 06:50



Mathematics, 29.05.2021 06:50


Mathematics, 29.05.2021 06:50

Physics, 29.05.2021 06:50

Mathematics, 29.05.2021 06:50

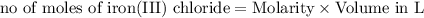
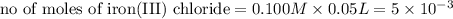
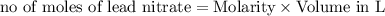
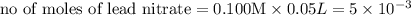
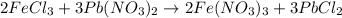
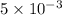 moles of lead nitrate react with
moles of lead nitrate react with 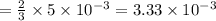 of ferric chloride.
of ferric chloride.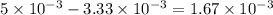 moles of ferric chloride will be left unreacted.
moles of ferric chloride will be left unreacted.

