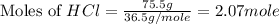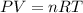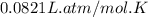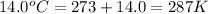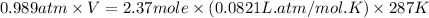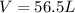Chemistry, 28.06.2019 19:30 AnastasiaJauregui
What is the mass of solid nh4cl formed when 75.5g of nh3 is mixed with an equal mass of hcl? what is the volume of the gas remaining, measured at 14.0c and 752 mmhg? what gas is it? the formula is nh3 (g) + hcl gas -> nh4cl solid

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2
You know the right answer?
What is the mass of solid nh4cl formed when 75.5g of nh3 is mixed with an equal mass of hcl? what i...
Questions


Mathematics, 25.05.2021 18:20

Chemistry, 25.05.2021 18:20

Geography, 25.05.2021 18:20

Social Studies, 25.05.2021 18:20


Mathematics, 25.05.2021 18:20

Mathematics, 25.05.2021 18:20

English, 25.05.2021 18:20



Mathematics, 25.05.2021 18:20

Physics, 25.05.2021 18:20


Mathematics, 25.05.2021 18:20

Mathematics, 25.05.2021 18:20




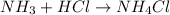
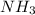 and HCl.
and HCl.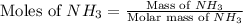
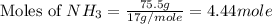
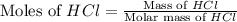
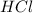 = 36.5 g/mole
= 36.5 g/mole