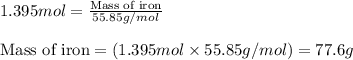Chemistry, 28.06.2019 20:30 Wondersixeleven
When 125 grams of feo react with 25.0 grams of al, how many grams of fe can be produced? feo + al → fe + al2o3 25.9 g fe 38.7 g fe 50.9 g fe 77.6 g fe

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 22.06.2019 21:30
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3

Chemistry, 23.06.2019 00:20
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
When 125 grams of feo react with 25.0 grams of al, how many grams of fe can be produced? feo + al →...
Questions

Mathematics, 16.10.2020 07:01

Mathematics, 16.10.2020 07:01

Social Studies, 16.10.2020 07:01


English, 16.10.2020 07:01

Chemistry, 16.10.2020 07:01

Mathematics, 16.10.2020 07:01


Mathematics, 16.10.2020 07:01


Advanced Placement (AP), 16.10.2020 07:01

Mathematics, 16.10.2020 07:01

Mathematics, 16.10.2020 07:01



History, 16.10.2020 07:01

Mathematics, 16.10.2020 07:01



Mathematics, 16.10.2020 07:01

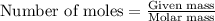 .....(1)
.....(1)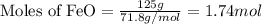
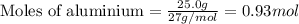
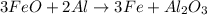
 of FeO
of FeO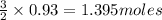 of iron metal
of iron metal