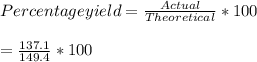Chemistry, 29.06.2019 02:00 101EXPERIENCE
Consider the following reaction: 2h2s (g) + 3o2 (g) 2so2 (g) + 2h2o (g) if o2 was the excess reagent, 8.3 mol of h2s were consumed, and 137.1 g of water were collected after the reaction has gone to completion, what is the percent yield of the reaction? show your work.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:50
How does a scientist the government? a. the scientist tells people in society what to do. b. the scientist determines the policies that the government spends money on. c. the scientist provides unbiased information to the government. d. the scientist makes laws based on his or her research results.
Answers: 1

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1


Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1
You know the right answer?
Consider the following reaction: 2h2s (g) + 3o2 (g) 2so2 (g) + 2h2o (g) if o2 was the excess reag...
Questions

Physics, 24.08.2019 10:00

Social Studies, 24.08.2019 10:00



Mathematics, 24.08.2019 10:00




Mathematics, 24.08.2019 10:00

Mathematics, 24.08.2019 10:00



Biology, 24.08.2019 10:00



Mathematics, 24.08.2019 10:00

Mathematics, 24.08.2019 10:00