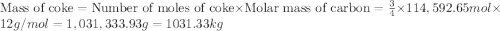The reduction of iron(iii) oxide (fe2o3) to pure iron during the first step of steelmaking, 2fe2o3(s)→ 4fe(s)+ 3o2(g) is driven by the high-temperature combustion of coke, a purified form of coal: c(s)+ o2(g)→ co2(g) suppose at the temperature of a blast furnace the gibbs free energies of formation δgf of co2 and fe2o3 are −429./kjmol and −835./kjmol, respectively. calculate the minimum mass of coke needed to produce 6400.kg of pure iron. round your answer to 2 significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 23.06.2019 10:30
Fill in the blanks for the following statements: the rms speed of the molecules in a sample of h2 gas at 300 k will be times larger than the rms speed of o2 molecules at the same temperature, and the ratio µrms (h2) / µrms (o2) with increasing temperature. a not enough information is given to answer this question b sixteen, will not change c four, will not change d four, will increase e sixteen, will decrease
Answers: 2
You know the right answer?
The reduction of iron(iii) oxide (fe2o3) to pure iron during the first step of steelmaking, 2fe2o3(s...
Questions


Mathematics, 19.06.2021 09:10

Physics, 19.06.2021 09:10







Mathematics, 19.06.2021 09:20


Chemistry, 19.06.2021 09:20

Business, 19.06.2021 09:20

Mathematics, 19.06.2021 09:20





Mathematics, 19.06.2021 09:20

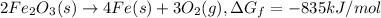 ...(1)
...(1)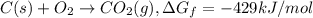 ...(2)
...(2)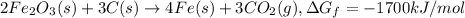 ...(3)
...(3)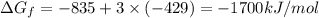
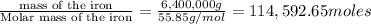
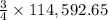 moles of coke.
moles of coke.