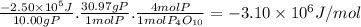Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2
You know the right answer?
When 10.00 g of phosphorus is burned in o2(g) to form p4o10(s), enough heat is generated to raise th...
Questions







Arts, 22.06.2019 00:30

World Languages, 22.06.2019 00:30

Business, 22.06.2019 00:30



History, 22.06.2019 00:30

Social Studies, 22.06.2019 00:30

Mathematics, 22.06.2019 00:30



English, 22.06.2019 00:30

Mathematics, 22.06.2019 00:30


Mathematics, 22.06.2019 00:30