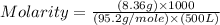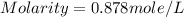Chemistry, 29.06.2019 12:00 Wintersavannah
Determine the molarity of a solution made by dissolving 8.36g of mgcl2 in water where the final volume of the solution is 500.0ml

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
19. at high pressures, how does the volume of a real gas compare with the volume of an ideal gas under the same conditions, and why? eman- it is much less because real gas partides are not moving. there is no difference because the gas laws are always obeyed. it is much less because at high pressures the temperature drops. it is much greater because real gas partides take up space.
Answers: 1

Chemistry, 22.06.2019 02:10
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

You know the right answer?
Determine the molarity of a solution made by dissolving 8.36g of mgcl2 in water where the final volu...
Questions

English, 24.01.2022 14:00



Mathematics, 24.01.2022 14:00


Computers and Technology, 24.01.2022 14:00

Mathematics, 24.01.2022 14:00


Mathematics, 24.01.2022 14:00


Mathematics, 24.01.2022 14:00


Computers and Technology, 24.01.2022 14:00

Mathematics, 24.01.2022 14:00


Biology, 24.01.2022 14:00


Mathematics, 24.01.2022 14:00


History, 24.01.2022 14:00

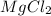 = 8.36 g
= 8.36 g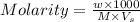
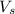 = volume of solution in liter
= volume of solution in liter