
Identify the equations that represent reactions that could occur. select all that apply. a) bacl2(aq) + na2so4(aq) ==> baso4(s) + 2nacl(aq) b) nabr(aq) + kcl(aq) ==> nacl(aq) + kcl(aq) c) nh4no3(aq) + agclo3(aq) ==> agno3(aq) + nh4clo3(aq) d) hch3coo(aq) + nahco3(aq) ==> nach3coo(aq) + co2(g) + h2o(l) e) 2naoh(aq) + cacl2(aq) ==> 2nacl(aq) + ca(oh)2(s)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1

Chemistry, 22.06.2019 20:30
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1
You know the right answer?
Identify the equations that represent reactions that could occur. select all that apply. a) bacl2(aq...
Questions

Mathematics, 27.10.2020 06:20

Mathematics, 27.10.2020 06:20

Chemistry, 27.10.2020 06:20

Mathematics, 27.10.2020 06:20


Law, 27.10.2020 06:20


Mathematics, 27.10.2020 06:20


English, 27.10.2020 06:20

Mathematics, 27.10.2020 06:20

Mathematics, 27.10.2020 06:20

Mathematics, 27.10.2020 06:20

Mathematics, 27.10.2020 06:20

History, 27.10.2020 06:20


Mathematics, 27.10.2020 06:20

Biology, 27.10.2020 06:20

Mathematics, 27.10.2020 06:20

 : This reaction occurs as there is a formation of white precipitate of barium sulfate.
: This reaction occurs as there is a formation of white precipitate of barium sulfate.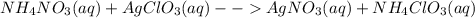 : This reaction does not occur because all the ions just remain as spectator ions in the solution as the products are aqueous too.
: This reaction does not occur because all the ions just remain as spectator ions in the solution as the products are aqueous too.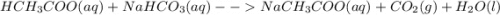 :This reaction can be observed as this proceeds via the formation of gas bubbles of carbon dioxide.
:This reaction can be observed as this proceeds via the formation of gas bubbles of carbon dioxide. :This reaction occurs as there is a formation of white precipitate of barium hydroxide.
:This reaction occurs as there is a formation of white precipitate of barium hydroxide.


