
Chemistry, 29.06.2019 17:00 officialalex6330
Both stearic acid and sucrose are molecular solids, but one is polar and the other is non polar. compare the solubility of the two compounds in water and in hexane to determine which is which

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 02:30
List four observations that indicate that a chemical reaction may be taking place
Answers: 1

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2
You know the right answer?
Both stearic acid and sucrose are molecular solids, but one is polar and the other is non polar. com...
Questions


Mathematics, 29.09.2020 15:01

Mathematics, 29.09.2020 15:01

Mathematics, 29.09.2020 15:01



Chemistry, 29.09.2020 15:01

Chemistry, 29.09.2020 15:01


Business, 29.09.2020 15:01


Mathematics, 29.09.2020 15:01



Advanced Placement (AP), 29.09.2020 15:01

English, 29.09.2020 15:01


Computers and Technology, 29.09.2020 15:01


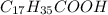 . The hydrophobic part is this chain is made up of 17 carbon atom and hydrophilic part is only of made up of carboxylic group due to which it won't get dissolve in water.Which means that stearic acid will get dissolve in non polar solvent that is hexane. Hence, stearic acid is non polar molecule.
. The hydrophobic part is this chain is made up of 17 carbon atom and hydrophilic part is only of made up of carboxylic group due to which it won't get dissolve in water.Which means that stearic acid will get dissolve in non polar solvent that is hexane. Hence, stearic acid is non polar molecule.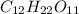 .Due to presence of polar hydroxyl(-OH) groups in molecule , molecule will get easily dissolve in polar solvent that is water. Since,the hydrophobic part in the molecules are covered by the hydrophilic part due to which it gets easily dissolve in water. Hence, the sucrose in polar molecule.
.Due to presence of polar hydroxyl(-OH) groups in molecule , molecule will get easily dissolve in polar solvent that is water. Since,the hydrophobic part in the molecules are covered by the hydrophilic part due to which it gets easily dissolve in water. Hence, the sucrose in polar molecule.

