Chemistry, 29.06.2019 22:30 leelee8335
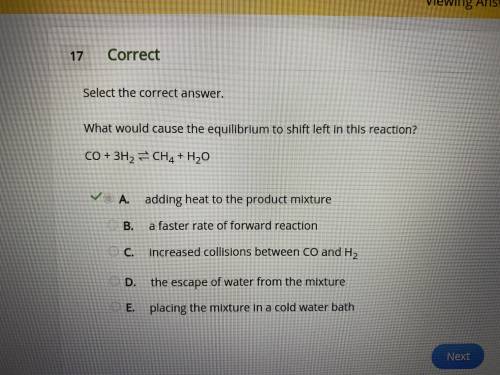
What would cause the equilibrium to shift left in this reaction? co + 3h2 ⇌ ch4 + h2o a. adding heat to the product mixture b. a faster rate of forward reaction c. increased collisions between co and h2 d. the escape of water from the mixture e. placing the mixture in a cold water bath

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Water (2510 g ) is heated until it just begins to boil. if the water absorbs 5.09×105 j of heat in the process, what was the initial temperature of the water?
Answers: 3

Chemistry, 22.06.2019 12:00
Marcel just purchased 1.69 grams of iron fillings in order to make living putty for his 6 year old niece. how many moles of iron are made in his sample?
Answers: 1

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3
You know the right answer?
What would cause the equilibrium to shift left in this reaction? co + 3h2 ⇌ ch4 + h2o a. adding hea...
Questions

English, 16.06.2021 21:30

Biology, 16.06.2021 21:30



Mathematics, 16.06.2021 21:30



Mathematics, 16.06.2021 21:30

Computers and Technology, 16.06.2021 21:30



Mathematics, 16.06.2021 21:30

Mathematics, 16.06.2021 21:30

History, 16.06.2021 21:30

Mathematics, 16.06.2021 21:30

Mathematics, 16.06.2021 21:30

English, 16.06.2021 21:30


Chemistry, 16.06.2021 21:30

Biology, 16.06.2021 21:30