Chemistry, 29.06.2019 22:30 annyarias1036
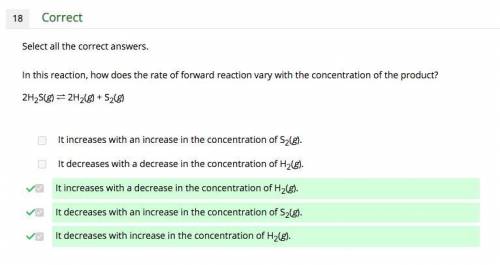
In this reaction, how does the rate of forward reaction vary with the concentration of the product? 2h2s(g) ⇌ 2h2(g) + s2(g) a. it increases with an increase in the concentration of s2(g). b. it decreases with a decrease in the concentration of h2(g). c. it increases with a decrease in the concentration of h2(g). d. it decreases with an increase in the concentration of s2(g). e. it decreases with increase in the concentration of h2(g). can choose more than one

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3
You know the right answer?
In this reaction, how does the rate of forward reaction vary with the concentration of the product?...
Questions

Mathematics, 19.01.2021 22:20

Mathematics, 19.01.2021 22:20




Chemistry, 19.01.2021 22:20

Mathematics, 19.01.2021 22:20


Spanish, 19.01.2021 22:20

Mathematics, 19.01.2021 22:20




History, 19.01.2021 22:20

Mathematics, 19.01.2021 22:20