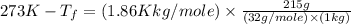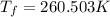Chemistry, 29.06.2019 23:30 FunnySkittle
Asolution is prepared by dissolving 215 grams of methanol, ch3oh, in 1000. grams of water. what is the freezing point of this solution? [the freezing point depression constant for water is 1.86°c/mole solute in 1000g of water]

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2


Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 23.06.2019 00:30
Ok, so i have 2 questions. try to answer them both: (the topic is fire) 1) how can you represent the chemical reaction of fire? 2) what kind of bond is formed in this chemical reaction
Answers: 3
You know the right answer?
Asolution is prepared by dissolving 215 grams of methanol, ch3oh, in 1000. grams of water. what is t...
Questions



English, 29.06.2019 17:20

Physics, 29.06.2019 17:20







Mathematics, 29.06.2019 17:20



Mathematics, 29.06.2019 17:20




Physics, 29.06.2019 17:20

Mathematics, 29.06.2019 17:20

Mathematics, 29.06.2019 17:20

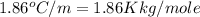
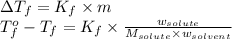
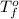 = freezing point of water =
= freezing point of water = 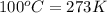
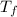 = freezing point of solution
= freezing point of solution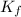 = freezing point constant
= freezing point constant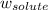 = mass of solute
= mass of solute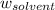 = mass of solvent
= mass of solvent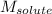 = molar mass of solute
= molar mass of solute