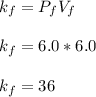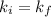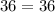Acertain gas is present in a 12.0l cylinder at 3.0 atm pressure. if the pressure is increased to 6.0 atm, the volume of the gas decreases to 6.0l. find the two constants ki, the initial value of k, and kf, the final value of k, to verify if whether the gas obeys boyle’s law.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:10
Why is the vapor pressure of a warm lake higher than the vapor pressure of a cold lake? o a. warm water has a greater heat of vaporization. ob. warm water evaporates more quickly. cool water evaporates more quickly. od. cool water has a greater heat of vaporization.
Answers: 1

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 08:30
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1
You know the right answer?
Acertain gas is present in a 12.0l cylinder at 3.0 atm pressure. if the pressure is increased to 6.0...
Questions


Mathematics, 30.10.2019 00:31


Geography, 30.10.2019 00:31

English, 30.10.2019 00:31

Mathematics, 30.10.2019 00:31

Biology, 30.10.2019 00:31

Mathematics, 30.10.2019 00:31

English, 30.10.2019 00:31

Mathematics, 30.10.2019 00:31


Mathematics, 30.10.2019 00:31


History, 30.10.2019 00:31





Mathematics, 30.10.2019 00:31

Computers and Technology, 30.10.2019 00:31

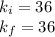
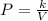
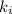 :
: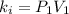
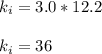
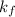 :
: