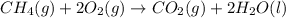Chemistry, 30.06.2019 00:00 alexwlodko
The combustion of methane gas (ch4) forms co2(g)+ h2o(ℓ). calculate the heat produced by burning 1.9 mol of the methane gas. the standard molar enthalpy of combustion of methane is 890.2kj/mol. answer in units of kj

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3

Chemistry, 22.06.2019 10:50
How many grams of oxygen gas are contained in a 15 l sample at 1.02 atm and 28°c? show your work.
Answers: 1

Chemistry, 23.06.2019 07:00
Explain what happened when the storm surges from hurricanes reached the gulf coast
Answers: 1

Chemistry, 23.06.2019 13:00
What type of reaction is this equation c2h5s + o2 > co2 + h2o + so2
Answers: 2
You know the right answer?
The combustion of methane gas (ch4) forms co2(g)+ h2o(ℓ). calculate the heat produced by burning 1.9...
Questions



Mathematics, 03.11.2020 18:50




Computers and Technology, 03.11.2020 18:50

Mathematics, 03.11.2020 18:50

Mathematics, 03.11.2020 18:50



History, 03.11.2020 18:50



Computers and Technology, 03.11.2020 18:50


Mathematics, 03.11.2020 18:50


Mathematics, 03.11.2020 18:50