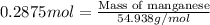Chemistry, 30.06.2019 00:00 TH3L0N3W0LF
Considering the limiting reactant, what is the mass of manganese produced from 25.0 g of manganese(iv) oxide (86.94 g/mol) and 25.0 g of aluminum metal? 3 mno2(l) + 4 al(l) 3 mn(l) + 2 al2o3(s)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Agas has a volume of 0.7 l at 300 mmhg. what would be the new volume at 900 mmhg
Answers: 1

Chemistry, 22.06.2019 00:00
The p sub shell can hold up to 8 electrons in an atom. true or false?
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1
You know the right answer?
Considering the limiting reactant, what is the mass of manganese produced from 25.0 g of manganese(i...
Questions


Mathematics, 31.12.2020 17:30

English, 31.12.2020 17:30

Mathematics, 31.12.2020 17:30

English, 31.12.2020 17:30

Physics, 31.12.2020 17:30


Social Studies, 31.12.2020 17:30


History, 31.12.2020 17:30

Mathematics, 31.12.2020 17:30

English, 31.12.2020 17:30

Mathematics, 31.12.2020 17:30


Mathematics, 31.12.2020 17:30

Social Studies, 31.12.2020 17:30




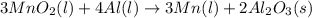
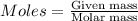 .....(1)
.....(1)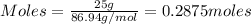
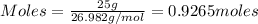
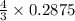 = 0.3833 moles of aluminium.
= 0.3833 moles of aluminium.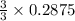 = 0.2875 moles of manganese.
= 0.2875 moles of manganese.