
Chemistry, 30.06.2019 02:30 brooke3493
Which statement is true? a) breaking a chemical bond releases energy. b) when energy is absorbed, the reaction is said to be endothermic and â†h is negative (–). c) bond dissociation energies are always positive numbers. d) the stronger the bond, the lower its bond dissociation energy. 2. based on the reaction, n2 + o2 → 2 no â†h = 43.2 kcal which statement is true? a) 43.2 kcal are consumed when 14.00 g of n2 reacts. b) 43.2 kcal are produced when 1.00 g of o2 reacts. c) 43.2 kcal are produced when 1.00 mole of o2 reacts. d) 43.2 kcal are consumed when 2.00 moles of no is produced.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Apeak with a retention time of 407 s has a width at half-height (w1/2) of 7.6 s. a neighboring peak is eluted 17 s later with a w1/2 of 9.4 s. a compound that is known not to be retained was eluted in 2.5 s. the peaks are not baseline resolved. how many theoretical plates would be needed to achieve a resolution of 1.5?
Answers: 2

Chemistry, 22.06.2019 23:00
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1

Chemistry, 23.06.2019 04:00
The movement of tectonic plates and in two locations is described below: location a: tectonic played push together location b: tectonic plates push apart
Answers: 1

You know the right answer?
Which statement is true? a) breaking a chemical bond releases energy. b) when energy is absorbed, t...
Questions



Mathematics, 21.06.2019 15:00

Mathematics, 21.06.2019 15:00


Mathematics, 21.06.2019 15:00

Mathematics, 21.06.2019 15:00

Physics, 21.06.2019 15:00

English, 21.06.2019 15:00


Mathematics, 21.06.2019 15:00



Mathematics, 21.06.2019 15:00


English, 21.06.2019 15:00


Spanish, 21.06.2019 15:00

Health, 21.06.2019 15:00

Geography, 21.06.2019 15:00

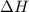 will be positive not negative.
will be positive not negative.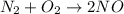
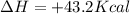
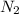 is reacted.
is reacted.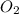 is reacted.
is reacted.

