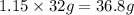Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3
You know the right answer?
Given the following equation: 2h2o --> 2h2 +o2 what mass of oxygen would form from 2.30 moles o...
Questions

Mathematics, 23.12.2019 16:31


Biology, 23.12.2019 16:31

Spanish, 23.12.2019 16:31

Chemistry, 23.12.2019 16:31


Health, 23.12.2019 16:31

English, 23.12.2019 16:31


Mathematics, 23.12.2019 16:31

Biology, 23.12.2019 16:31

History, 23.12.2019 16:31

Mathematics, 23.12.2019 16:31





Mathematics, 23.12.2019 16:31

Mathematics, 23.12.2019 16:31

Mathematics, 23.12.2019 16:31

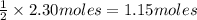
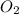 is 32 g/mol
is 32 g/mol