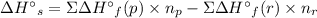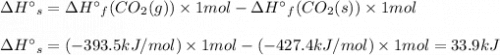Chemistry, 30.06.2019 07:00 ineedhelp2285
Use standard enthalpies of formation to calculate the change in enthalpy for dry ice sublimation. (the î´hâf for co2(s) is - 427.4kj/mol).

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Diamond, graphite, and fullerenes share what property? a. they are all made of carbon (c) bonded to a metal. b. their shape. c. they are all made of carbon (c). d. they are all good conductors.
Answers: 1


Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1
You know the right answer?
Use standard enthalpies of formation to calculate the change in enthalpy for dry ice sublimation. (t...
Questions

Physics, 23.10.2020 20:10

Mathematics, 23.10.2020 20:10

Mathematics, 23.10.2020 20:10






Mathematics, 23.10.2020 20:10


Mathematics, 23.10.2020 20:10

Mathematics, 23.10.2020 20:10

Law, 23.10.2020 20:10

Computers and Technology, 23.10.2020 20:10


Mathematics, 23.10.2020 20:10




Mathematics, 23.10.2020 20:10