
Chemistry, 30.06.2019 11:00 Imamdiallo18
In the balanced equation , cs2 + 3o2 = co2 + 2so2 , how many mol of o2 would react with 34.5 mol of co2?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 23.06.2019 00:20
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1

Chemistry, 23.06.2019 02:20
Why dose heating increase the speed at which a solution dissolved in water
Answers: 1
You know the right answer?
In the balanced equation , cs2 + 3o2 = co2 + 2so2 , how many mol of o2 would react with 34.5 mol of...
Questions

Health, 27.10.2020 21:10


Mathematics, 27.10.2020 21:10




Mathematics, 27.10.2020 21:10

Geography, 27.10.2020 21:10


English, 27.10.2020 21:10




Mathematics, 27.10.2020 21:10


Biology, 27.10.2020 21:10


Social Studies, 27.10.2020 21:10


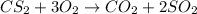
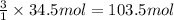 oxygen gas.
oxygen gas.

