
Chemistry, 30.06.2019 11:00 aaliyahnv07
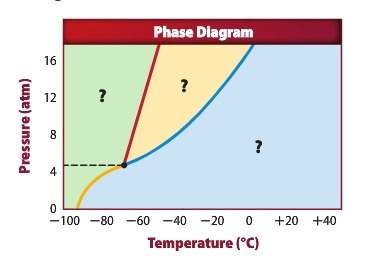
Using the diagram above answer the following questions: a) the green section of the graph represents: b) the yellow section of the graph represents: c) the blue section of the graph represents: d) what is the phase indicated by the yellow line if you are removing energy: e) at 12.00 atm and -40°c, what phase is present:

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Your answer should have the same number or significant figures as a he starting measurement. 3201 ml =
Answers: 2

Chemistry, 21.06.2019 22:30
1.aluminum chloride (alcl3), and sodium hydroxide (naoh) can react to form aluminum hydroxide (al(oh)3) and sodium chloride (nacl). you have 13.4 g of aluminum chloride and 10.0 g of sodium hydroxide. answer the following questions: •what is the balanced equation for this reaction? •if you use all 13.4 g of aluminum chloride, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •if you use all 10.0 g of sodium hydroxide, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •how many grams of aluminum hydroxide will actually be made? which reagent is limiting? explain your answer.
Answers: 1

Chemistry, 21.06.2019 22:50
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 21.06.2019 23:30
Two atoms interact with each other as shown by the equation. complete the equation by filling in the missing parts. 1 2 3 4 5 h he li
Answers: 2
You know the right answer?
Using the diagram above answer the following questions: a) the green section of the graph represen...
Questions


History, 30.01.2020 14:04



History, 30.01.2020 14:04

Mathematics, 30.01.2020 14:04






Mathematics, 30.01.2020 14:04




Mathematics, 30.01.2020 14:04

Biology, 30.01.2020 14:04

Mathematics, 30.01.2020 14:04


English, 30.01.2020 14:04



