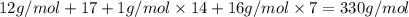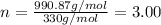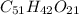Chemistry, 30.06.2019 11:30 megandalolipop
Aformer high school teacher was discovered making meth, and the dea recently analyzed some confiscated material from a drug bust he was associated with. they found a compound with the empirical formula c17h14o7 that has a molar mass of 990.87 g/mol. what is the value of n (the multiplier) necessary to find the molecular formula?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3

Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

You know the right answer?
Aformer high school teacher was discovered making meth, and the dea recently analyzed some confiscat...
Questions

History, 21.08.2019 07:50




Biology, 21.08.2019 07:50

Geography, 21.08.2019 07:50


Social Studies, 21.08.2019 07:50


Mathematics, 21.08.2019 07:50



History, 21.08.2019 07:50



History, 21.08.2019 07:50

Social Studies, 21.08.2019 07:50

Geography, 21.08.2019 07:50


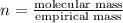
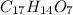 :
: